Definition
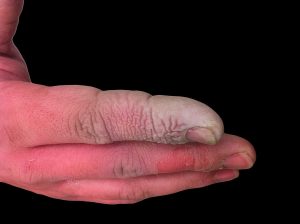
Hydrofluoric acid, also known as HF, is a strong and highly corrosive acid that is widely used in various industries. Its versatility and effectiveness in many applications make it an essential chemical compound. However, hydrofluoric acid presents significant safety and environmental concerns due to its toxic nature. This essay aims to explore the safety aspects of using hydrofluoric acid and highlight its potential environmental impacts.
Safety first
Firstly, one must be well-informed about the safety measures and protocols when handling hydrofluoric acid. HF is particularly hazardous due to its ability to permeate the skin and penetrate tissues, which can lead to severe burns and tissue damage. It is crucial to wear protective clothing, such as gloves, goggles, and lab coats, to minimize contact with the acid. Additionally, hydrofluoric acid should be stored in appropriate containers and in well-ventilated areas to avoid accidental spills and inhalation of fumes.
Environmental impacts
Moreover, it is crucial to dispose of hydrofluoric acid properly to minimize its detrimental environmental impacts. HF is highly reactive and can release harmful compounds into the air and water if not handled correctly. The acid should be neutralized before disposal to minimize its corrosive effects. Special attention should be given to preventing its release into water bodies and wastewater systems, as it can harm aquatic life and contaminate drinking water sources.
Industrial use
Furthermore, in industries where hydrofluoric acid is used in large quantities, it is crucial to have comprehensive safety protocols in place. Employees should undergo regular training, ensuring they understand the potential risks associated with working with HF and the appropriate safety measures. Employers must provide adequate ventilation systems, spillage response equipment, and eyewash stations to ensure the safety of their employees and reduce accidental exposures.
In addition to safety concerns, hydrofluoric acid’s environmental impact should also be carefully considered. The acid can contribute to air pollution through the release of toxic gases during its manufacturing, storage, and use. These emissions can lead to respiratory problems and other health issues in nearby communities. Strategies should be implemented to control and reduce air emissions, such as installing scrubbers and filters to capture and contain HF fumes.
Disposal
Furthermore, the disposal of hydrofluoric acid waste should be closely monitored to prevent environmental contamination. Companies should adhere to strict guidelines and regulations regarding the handling and disposal of HF waste. Adequate treatment and proper storage facilities should be in place to prevent the acid from leaching into the soil and underground water sources. Regular inspections and monitoring should be carried out to ensure compliance with environmental standards and prevent any potential harm to ecosystems.
Conclusion
In conclusion, hydrofluoric acid is a potent chemical compound widely used in various industries. However, its usage poses significant safety risks to both individuals and the environment. Ensuring proper safety measures are in place, such as providing personal protective equipment and adequate training, is crucial to minimize accidental exposure. Additionally, appropriate storage, disposal, and treatment methods are essential to reduce the environmental impact of hydrofluoric acid usage. By implementing comprehensive safety protocols and adhering to strict environmental regulations, we can mitigate the potential hazards posed by this corrosive acid.