Hydrofluoric acid
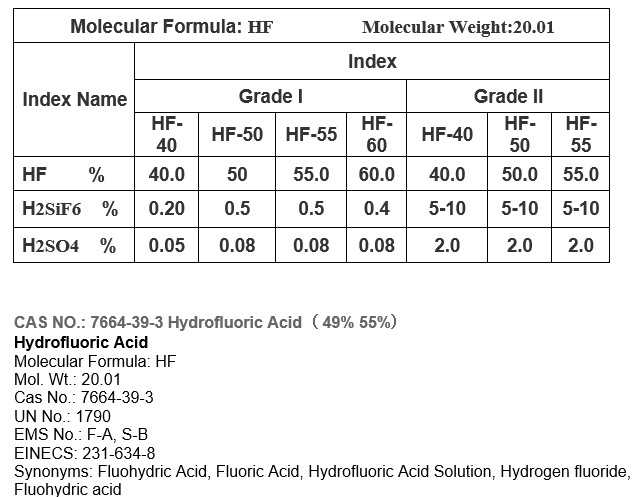
Hydrofluoric acid, also known as HF, is a highly corrosive and toxic acid. It is composed of hydrogen and fluorine, which are both highly reactive elements. The molecular formula for hydrofluoric acid is HF, indicating that it consists of one hydrogen atom bonded to one fluorine atom.
Applications
This product finds various applications due to its unique properties. One of the major uses of hydrofluoric acid is in the production of fluorine-containing compounds. These compounds are widely used in industries such as pharmaceuticals, chemicals, and electronics. This product is also employed in the glass etching process where it acts as an etchant, allowing intricate designs to be imprinted on glass surfaces. Furthermore, it is used for cleaning and rust removal due to its ability to dissolve silicate minerals and metal oxides.
Safety
Despite its numerous applications, This product can be extremely dangerous and requires careful handling. One of the most notable hazards associated with hydrofluoric acid is its ability to penetrate the skin and attack deep tissues. Even small exposures can lead to severe burns and exposure to high concentrations can result in fatal health consequences. Therefore, it is crucial to exercise caution and follow strict safety protocols when working with hydrofluoric acid.
Other applications
In addition to its direct applications, This product is also used in various industrial processes such as oil refining, steel pickling, and aluminum production. It is particularly useful in oil refining for removing impurities from petroleum products. This productacts as a catalyst in the alkylation process, enhancing the octane rating of gasoline. Furthermore, it plays a vital role in the production of aluminum by facilitating the removal of impurities and improving the quality of the final product.

Conclusion
In conclusion, This product is a highly reactive and corrosive acid with the molecular formula HF. It has a wide range of applications, including the production of fluorine-containing compounds, glass etching, cleaning, and industrial processes such as oil refining and aluminum production. Despite its utility, This product poses significant health risks and requires careful handling to avoid severe burns and other health issues. The proper understanding and cautious use of hydrofluoric acid are essential for ensuring its safe and effective application in various industries.