- In addition to drugs and polymers, fluorine chemical products play an important role in materials science.
- For example, researchers use fluoride salts such as sodium fluoride and ammonium Bifluoride to modify the surface of metals and improve their corrosion resistance.
- Similarly, fluoride compounds are used as catalysts in various industrial processes
- which facilitate the synthesis of important chemicals and our ability to produce essential products such as refrigerants and lithium-ion batteries.
- In addition, the reactivity of fluorine allows its use in etching processes for semiconductor production, an integral step in the fabrication of advanced electronic devices.
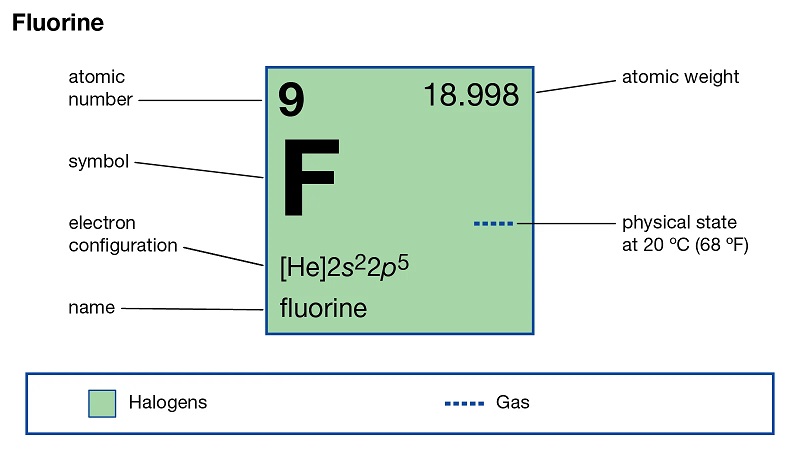
1. Introduction to Fluorine Derivatives in Health Products and Medicine
1.1 What are Fluorine Derivatives?
When it comes to health products and medicine, you probably don’t immediately think of fluorine derivatives. But these compounds play a crucial role in the development and effectiveness of various pharmaceuticals.
Fluorine derivatives are organic compounds that contain fluorine atoms. They are synthesized by incorporating fluorine into existing molecules, creating new compounds with unique properties and benefits. Fluorine, as an element, has special characteristics that make it a valuable component in pharmaceutical chemistry.