Definition
Caustic soda, also known as sodium hydroxide (NaOH), is an essential chemical compound used in various industrial processes. It is a strong base and highly reactive, making it a key ingredient in the production of various products like paper, soap, and detergents. This essay will describe the process involved in the production of caustic soda.
Brine
The production of caustic soda involves several steps, beginning with the electrolysis of salt. Saltwater, or brine, is first collected and purified. The purified brine is then pumped into large electrolysis cells. These cells consist of an anode and a cathode separated by a diaphragm. The anode is made of titanium and releases chlorine gas, while the cathode is made of steel and produces hydrogen gas and hydroxide ions.
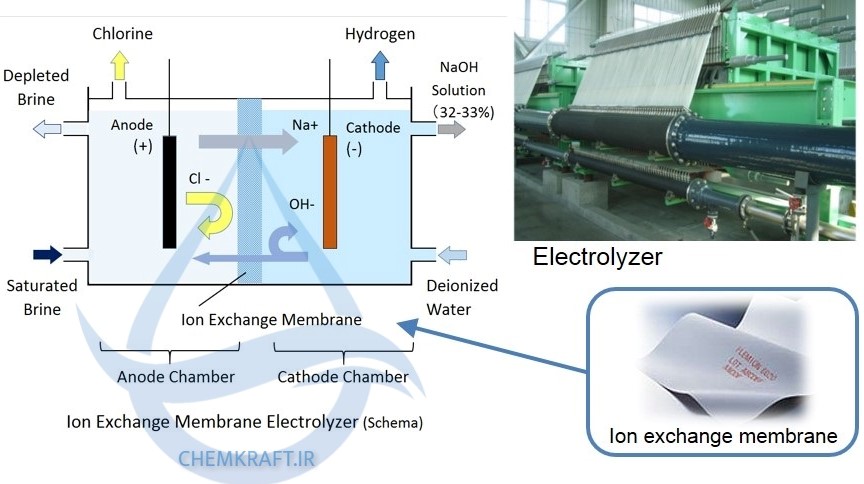
Electrolysis
As the direct current passes through the electrolysis cells, the hydroxide ions migrate to the cathode, creating a highly alkaline solution of sodium hydroxide. The hydrogen gas is then collected and typically used in other industrial processes. Simultaneously, chlorine gas is released at the anode and collected for various applications, such as the production of PVC and disinfectants.
Concentration
After the electrolysis process, the caustic soda solution is concentrated to remove excess water. One common method for concentration is evaporation in multiple-effect evaporators. These evaporators use steam to heat the solution, causing the water to evaporate while the sodium hydroxide remains in the concentrated solution. This process is repeated in several stages to achieve the desired concentration.
Purification
To further purify the solution, impurities like chloride ions and heavy metals are removed using various techniques such as ion exchange, precipitation, or membrane filtration. These purification steps ensure the final product meets the required quality standards and eliminates any potential contaminants that could affect the end-use applications of caustic soda.
Making flakes
Once the solution is concentrated and purified, it is further processed into different forms based on customer requirements. One common form is solid flakes or pellets, which are obtained by crystallizing the concentrated solution and drying the crystals. Another form is a liquid solution, which is stored in specified containers for easy transportation and use.
Energy consumption
It is important to note that the production of caustic soda is a highly energy-intensive process. The electrolysis of salt consumes a significant amount of electrical energy, making energy efficiency a crucial factor in the overall production process. Efforts are continually being made to improve the energy efficiency of caustic soda production through the implementation of advanced technologies and practices.
Final word
In conclusion, the production of caustic soda involves an intricate process that starts with the electrolysis of saltwater, followed by concentration, purification, and final processing into different forms. The production process ensures the removal of impurities and guarantees the quality of the caustic soda obtained. As a fundamental chemical compound, caustic soda plays a vital role in multiple industries, and the constant improvements in the production process contribute to its widespread availability and effectiveness.










