Aluminum fluoride, also known as AlF3, is a versatile compound with numerous applications in various industries. This essay aims to provide an in-depth understanding of this chemical, including its chemical properties, production methods, applications, and potential health and environmental effects.
AlF3 as a fusing assistant for ceramic and the component of glaze
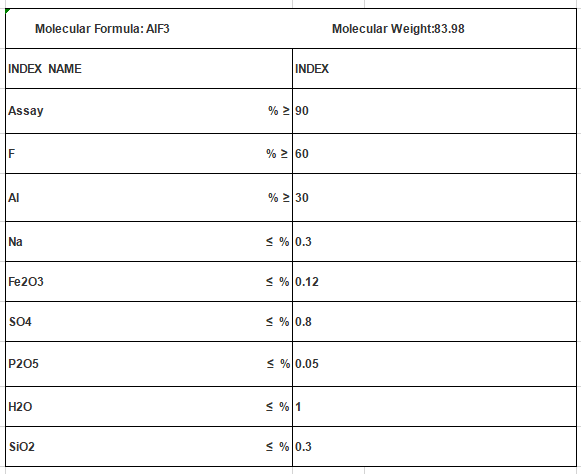
Molecular Formula: ALF3
Mol. Wt: 83.98
Cas No.: 7784-18-1
EINECS: 232-051-1
Physical state: White crystal or powde
Melting point: 1290 °C (lit.)
Boiling point: 1291 °C
Solubility: Slightly soluble in water. Sparingly soluble in acids and alkalies. Insoluble in Acetone
Regular package: 25kg per bags, or according to customer’s requirements
Storage: Keep container tightly closed in a dry and well-ventilated place. Do not store in glass
Transportation: Not dangerous goods
This chemical is a white, crystalline solid with a molecular formula of AlF3 and a molar mass of 82.00 g/mol. It is highly soluble in water and has a melting point of 1,291 °C. Its crystal structure comprises aluminum ions coordinated with six fluoride ions, forming octahedral complexes. This compound exhibits remarkable stability due to the strong bonds between aluminum and fluoride ions.
Production
The primary method of producing AlF3 involves the reaction between aluminum hydroxide and hydrofluoric acid. This process typically occurs in a heated stainless-steel reactor, where aluminum hydroxide reacts with hydrofluoric acid to form aluminum fluoride and water. The resultingthis chemical is then purified and dried to obtain the final product. Other production methods include the reaction of aluminum oxide with hydrogen fluoride or the fusion of aluminum metal and aluminum oxide with hydrogen fluoride gas.
Due to its excellent physical and chemical properties, aluminum fluoride finds applications in diverse industries. One of its major uses is as a flux in the production of aluminum. It lowers the melting point of alumina, promoting better conductivity and allowing better control over the refining process. Additionally, aluminum fluoride is utilized in the production of ceramics, glass, and enamel. It serves as an opacifier, giving these materials a white, opaque appearance. Furthermore, aluminum fluoride plays a crucial role in the manufacturing of insecticides, as a catalyst in organic synthesis reactions, and as an electrolyte additive in lithium-ion batteries.
Health and environmental effects
While aluminum fluoride has numerous advantageous applications, it is essential to consider potential health and environmental effects associated with its use. High exposure to this chemical may cause respiratory irritation, eye irritation, and skin burns. Prolonged ingestion or inhalation of AlF3 can have detrimental effects on the nervous system, leading to conditions such as Alzheimer’s disease. Moreover, improper disposal or release of this chemical can lead to environmental contamination, affecting aquatic ecosystems and potentially harming aquatic life.
In conclusion
aluminum fluoride is a versatile compound with significant uses across various industries. Its exceptional physical and chemical properties, such as high solubility and stability, enable its application in the production of aluminum, ceramics, glass, enamel, insecticides, catalysts, and lithium-ion batteries. However, it is crucial to handle it with care, considering its potential health effects on humans and environmental impact.






