NH4BF4
Ammonium Fluoroborate 98% CAS NO. : 13826-83-0
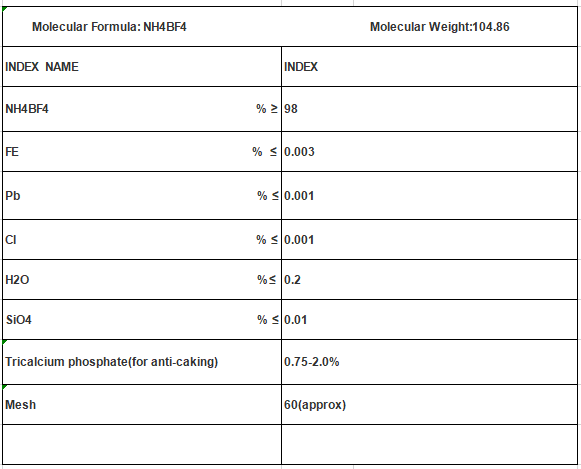
Molecular Formula: NH4BF4
Mol. Wt: 104.86
Cas No.: 13826-83-0
UN No.: 3260
EINECS: 237-531-4
Synonyms: Ammonium Tetrafluoroborate, Ammonium Fluoborate
Physical state: White powder
Melting point: 230°C
Relative density: 1.871 g/mL at 25 °C(lit.)
Regular package: 25kg/bag, or according to customer’s requirement
Storage: Moisture sensitive. Keep container tightly closed in a cool, dry and well-ventilated place
Transportation: Hazardous Chemical, Hazard Class: 8, Packing Group: III, UN No.: 3260

Ammonium Fluoroborate or ammonium tetrafluoroborate is a chemical compound that belongs to the family of fluoroborate salts. Its chemical formula is NH4BF4. This compound is widely used in various applications due to its unique properties and characteristics. In this essay, we will delve into discussing the formula and applications of this chemical.
The formula NH4BF4 represents Ammonium Fluoroborate, with NH4 representing the ammonium cation and BF4 denoting the fluoroborate anion. The ammonium cation is composed of one nitrogen atom bonded with four hydrogen atoms, whereas the fluoroborate anion consists of one boron atom bonded to four fluorine atoms. This composition creates a stable and versatile compound that finds applications in several fields.
One of the main applications of this compound is in the metal industry. It is commonly used as a fluxing agent during the soldering and brazing processes. The compound’s low melting point and ability to dissolve oxides facilitate the efficient joining of metal surfaces. Additionally, its use as a flux helps in preventing oxidation and promoting a clean metal surface, leading to strong and reliable bonds.
Applications
Ammonium Fluoroborate also finds application in the manufacturing of metal coatings and electroplating. Its unique properties make it an excellent additive in metal finishing processes. It helps in reducing the surface tension of metal solutions, improving wetting properties, and enhancing the overall quality of the coating or plating. Moreover, the compound’s ability to stabilize metal ions makes it an ideal component for electroplating baths, ensuring a smooth and uniform deposition of the metal.
Another noteworthy application of it is in the production of synthetic fluorine compounds. Due to its ability to release fluoride ions readily, it serves as an essential source of fluoride in numerous chemical reactions. These reactions involve the synthesis of various organic and inorganic compounds. Ammonium Fluoroborate’s role as a precursor contributes to the development of advanced materials, pharmaceuticals, and other important compounds.
In addition to its industrial uses, Ammonium Fluoroborate has some applications in the laboratory. It is often utilized as a reagent in analytical chemistry for testing cations such as mercury and lead. Its ability to form insoluble precipitates with these ions allows for their detection and separation from other compounds. This compound also acts as a catalyst in certain organic reactions, enabling the synthesis of complex molecules with high yields.
In conclusion
Ammonium Fluoroborate (NH4BF4) is a versatile compound with a variety of applications. Its formula consists of the ammonium cation and fluoroborate anion, allowing it to exhibit unique properties. From its uses in metal industry processes like soldering and electroplating to its role as a precursor in the synthesis of fluorine compounds, it plays a crucial role in various fields. Moreover, its applications extend to the laboratory, serving as a reagent and catalyst in analytical chemistry and organic synthesis. Overall, Ammonium Fluoroborate proves to be an essential compound with significant contributions to different industries and scientific advancements.