Anhydrous Potassium Fluoride
Anhydrous Potassium Fluoride, with the chemical formula KF, is an inorganic compound commonly used in various industrial applications. This compound is composed of a single potassium cation (K+) and fluoride anion (F-), and it is known for its unique properties and wide-ranging applications. In this essay, we will explore the formula, production, and applications of it.
Potassium Fluoride Anhydrous KF,CAS No.: 7789-23-3
Molecular Formula: KF
Mol. Wt: 58.10
CAS No.: 7789-23-3
UN No.: 1812
EMS No.: F-A, S-A
EINECS: 232-151-5
Synonyms: Potassium Fluoride
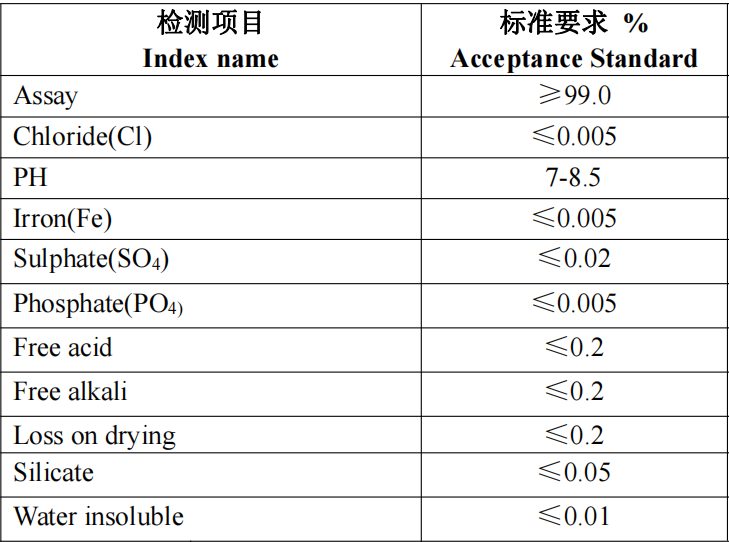
Physical state: white powder;
Melting point: 858 °C (lit.)
Package: 20kg plastic woven bags or according to customer’s requirements
Storage: Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep package sealed. It should be stored separately from acids and edible chemicals, and mixed storage should not be avoided.
Transportation: Hazardous chemical, Hazard class:6.1, Package group:III, UN
No.:1812
Definitions
The chemical formula , KF, represents the ratio of potassium ions to fluoride ions in the compound. It is formed by the ionic bond between the positively charged potassium ion and the negatively charged fluoride ion. This bond is quite strong, leading to the stability of the compound.
Production
The production of potassium fluoride involves a simple reaction between potassium carbonate (K2CO3) and hydrofluoric acid (HF). When these two compounds are mixed, a double displacement reaction occurs, resulting in the formation of it and carbon dioxide (CO2) as a byproduct. The reaction can be represented by the equation: K2CO3 + 2HF → 2KF + CO2 + H2O. The potassium fluoride obtained from this reaction can then be purified through various methods to obtain the desired grade required for specific applications.
Applications Anhydrous Potassium Fluoride
One of the most significant applications of this compound is its use in the glass industry. It is an essential component in the production of various types of glasses and glass products. It is commonly used as a fluxing agent to lower the melting point of the raw materials, thereby facilitating the manufacturing process. Additionally, potassium fluoride also acts as a refining agent, removing impurities and improving the clarity and transparency of the glass.
Anhydrous Potassium Fluoride also finds applications in the field of dentistry. It is commonly used in toothpaste and mouthwash products due to its ability to reduce tooth sensitivity and strengthen tooth enamel. The fluoride ions in the compound help to remineralize and protect the tooth surface, preventing dental decay and cavities.
Other applications
Another notable application of potassium fluoride is in the production of pesticides. It is used as a key ingredient in certain insecticides and fungicides due to its ability to disrupt the nervous system of pests. It is highly effective in controlling agricultural pests, providing farmers with an eco-friendly alternative to harmful chemical pesticides.
In conclusion
potassium fluoride, with the chemical formula KF, is an inorganic compound widely used in various industrial applications. It is produced through a reaction between potassium carbonate and hydrofluoric acid. The compound finds applications in the glass industry, dentistry, and as a pesticide. With its unique properties, this compound continues to play a crucial role in several industries, contributing to advancements in various fields.



