Definition
Sodium Fluorosilicate, also known as sodium silicofluoride, is a chemical compound with the formula Na2SiF6. It is a white, crystalline powder that is highly soluble in water. In this essay, we will explore the synonyms, formula, and applications of sodium silicofluoride.
Firstly, let’s examine the synonyms of Sodium Fluorosilicate. Apart from its scientific name, sodium silicofluoride, it is also known by other names such as sodium silicofluoride and sodium hexafluorosilicate. These synonyms highlight the composition and compound type, making it easier for scientists and chemists to communicate and refer to this chemical by its various names.
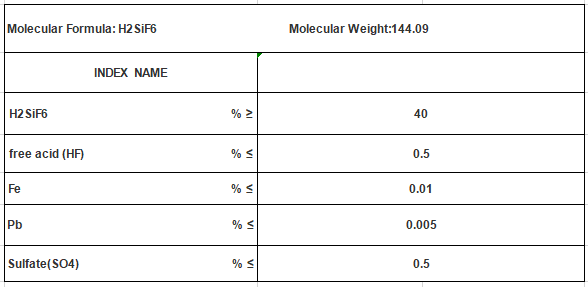
Hexafluorosilicic Acid H2SiF6 CAS No 16961-83-4
Molecular Formula: H2SiF6
Mol. Wt: 144.09
Cas No.: 16961-83-4
UN No.: 1778
EINECS: 241-034-8
Analysis
Moving on to the formula, this compound is represented by the chemical formula Na2SiF6. This formula displays the atomic composition and arrangement of the compound. It indicates that Sodium Fluorosilicate consists of two sodium (Na) atoms, one silicon (Si) atom, and six fluorine (F) atoms. The subscript numbers indicate the specific ratio of each element within the compound.
Now, let’s explore the applications of Sodium Fluorosilicate. This chemical compound finds numerous uses in various industries. One of the major applications of it is as a fluoride source for water fluoridation. Authorities add Sodium Fluorosilicate to public water supplies to promote dental health by preventing tooth decay and cavities. Manufacturers also use it in toothpaste and dental treatments for the same purpose.
Applications
In addition to dental care, Sodium Fluorosilicate is utilized in the preservation of wood. The compound acts as an insecticide and wood preservative, preventing deterioration caused by termites and decay fungi. It is also used in the refining of aluminum, where it helps remove impurities and reduce the melting point of the metal.
Furthermore, this chemical has diverse industrial applications. It serves as an important ingredient in the production of ceramics and glass. The compound assists in controlling the melting point and viscosity of glass, allowing for the formation of various glass products. Sodium Fluorosilicate is also used as a flux in metallurgical processes, helping to reduce the melting point of metal oxides and facilitating the removal of impurities.
Conclusion
In conclusion, Sodium Fluorosilicate, also known as sodium silicofluoride, or sodium hexafluorosilicate, is a chemical compound with the formula Na2SiF6. It finds wide applications in water fluoridation, dental care, wood preservation, aluminum refining, ceramics, glass production, and metallurgical processes. Handle Sodium Fluorosilicate with caution due to its toxicity, despite its potential benefits.Nevertheless, it remains an essential compound in various industries, contributing to improved oral health, durable wood structures, and the production of various materials.
Sodium Fluorosilicate (Na2SiF6): Applications and Global Production Landscape
Sodium Fluorosilicate, known as Na2SiF6, proves to be a compound with diverse applications and a notable presence in global production. This essay explores the extensive applications of Na2SiF6 and sheds light on the significant producing countries, emphasizing its importance across various industries.
Introduction:
Derived from the reaction between sodium chloride (NaCl) and hydrogen fluoride (HF), Na2SiF6 exhibits stability and solubility in water. This essay delves into the multifaceted applications of Sodium Fluorosilicate and examines the prominent producing countries, underlining its global significance.
Water Fluoridation:
A primary application of Na2SiF6 lies in water fluoridation. Controlled addition to water supplies ensures a consistent fluoride concentration, contributing to dental health by preventing tooth decay. This practice is globally adopted to enhance public health on a broad scale.
Metal Surface Treatment and Corrosion Inhibition:
In metal surface treatment, Na2SiF6 serves as a corrosion inhibitor. Applied as a protective layer, it enhances the durability of metal components, particularly in industries such as automotive and infrastructure where corrosion prevention is paramount.
Concrete and Cement Additive:
In the construction industry, Na2SiF6 acts as an additive in concrete and cement production. Its reaction with alkaline compounds in cement contributes to the development of robust and long-lasting infrastructure, enhancing the strength and durability of concrete structures.
Electroplating Enhancement:
Na2SiF6 plays a crucial role in electroplating processes, serving as an electrolyte. Its addition ensures a smoother and more uniform metal deposition, contributing to the production of high-quality coated products. This enhancement is particularly essential in industries like electronics and automotive.
Glass Etching Expertise:
In the glass industry, Na2SiF6 is employed as an etching agent. Its ability to selectively remove thin layers of glass facilitates the creation of intricate designs in decorative glassware, glass art, and electronic components. This controlled etching process is vital for achieving precise and aesthetically pleasing results.
Producing Countries:
The global production of Na2SiF6 is concentrated in several key countries. China holds a prominent position as the largest producer, contributing significantly to the global supply. The United States, Russia, and Brazil also stand out as noteworthy producers, actively participating in the global sodium fluorosilicate market.
China’s Dominance:
China dominates Na2SiF6 production due to its vast reserves of fluorspar, the primary raw material. The country’s well-established chemical industry and production infrastructure contribute to its capacity to meet both domestic and international demand.
United States:
The United States emerges as a significant producer of Na2SiF6, driven by its industrial applications and water fluoridation practices. The presence of fluorspar deposits and a robust chemical manufacturing sector supports the country’s role in global production.
Russia and Brazil:
These two countries also play crucial roles in Na2SiF6 production. Russia benefits from fluorspar resources, while Brazil leverages its position as a key player in the global mining and chemical industries to contribute to the production of Sodium Fluorosilicate.
Controlling Organizations and Safety:
Various international, national, and regional bodies regulate the responsible use of Na2SiF6 and other fluorine compounds. The International Council of Chemical Associations (ICCA) is a prominent organization that collaborates with stakeholders to ensure safety and responsible chemical use.
For specific regulations, individuals and organizations can refer to bodies such as the Occupational Safety and Health Administration (OSHA) in the United States and the European Chemicals Agency (ECHA) in Europe. These agencies provide comprehensive guidelines for the safe handling, storage, and disposal of chemicals, aligning with safety and environmental standards.
Conclusion:
In conclusion, Sodium Fluorosilicate (Na2SiF6) emerges as a compound with diverse applications, influencing industries from water treatment to metal surface treatment and beyond. China dominates the global production of this compound, with significant contributions from the United States, Russia, and Brazil. Regulatory bodies ensure the responsible use of Na2SiF6 by emphasizing safety and environmental standards. As research continues and industrial needs evolve, Sodium Fluorosilicate is likely to maintain its pivotal role in shaping various industrial processes and applications worldwide.



