Introduction
The chlor alkali production process is a vital industrial operation that encompasses the manufacturing of chlorine, sodium hydroxide (caustic soda), and hydrogen gas through electrolysis. This process plays a significant role in various industries such as chemical manufacturing, water treatment, and paper production. In this article, we will delve into the intricacies of chlor alkali production, with a particular focus on caustic production, concentration techniques, and the flaking process. Additionally, we will explore the environmental and safety considerations associated with this production process. Understanding these aspects will provide valuable insights into the efficient and sustainable production of chlor alkali products.
1. Chlor Alkali Production Process
1.1 Overview of Chlor Alkali Industry
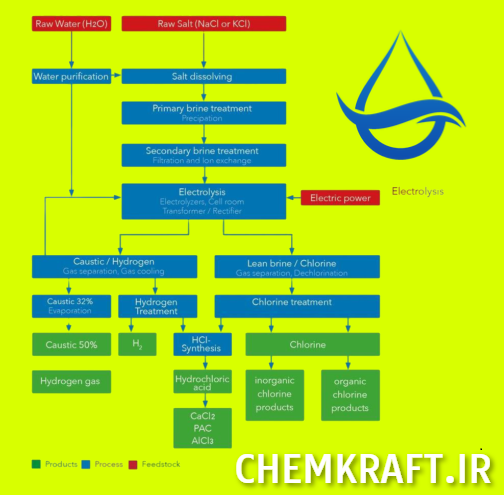
The chlor alkali industry plays a crucial role in various sectors, including manufacturing, water treatment, and chemical production. It involves the production of chlorine, sodium hydroxide (caustic), and hydrogen gas through an electrolysis process. These chemicals have widespread applications in industries such as paper, textiles, plastics, and pharmaceuticals.
1.2 Importance of Chlor Alkali Production
The importance of chlor alkali production cannot be overstated. Chlorine, a key product, is essential for disinfecting drinking water, wastewater treatment, and the production of thousands of everyday products. Sodium hydroxide, or caustic, is a versatile chemical used in the manufacturing of soaps, detergents, textiles, and cleaning agents. Additionally, hydrogen gas is used as a fuel source and plays a vital role in various industries.
2. Caustic Production: Overview and Importance
2.1 Definition and Properties of Caustic
Caustic, also known as sodium hydroxide (NaOH), is a highly corrosive, white solid. It is hygroscopic, meaning it absorbs moisture from the air, and has a strong alkaline nature. Caustic is an essential chemical in various industries, as it reacts with acids and other substances to form salts and water, making it useful in a wide range of applications.
2.2 Raw Materials Used in Caustic Production
The production of caustic involves the electrolysis of a solution called brine, which is primarily composed of sodium chloride (NaCl) and water (H2O). Brine is readily available as a natural resource or can be generated as a byproduct of other chemical processes.
2.3 Electrolysis Process for Caustic Production
The production of caustic involves an electrolysis process where a direct electric current is passed through the brine solution. This process takes place in specially designed electrolytic cells called diaphragm cells, membrane cells, or mercury cells. During electrolysis, the sodium chloride is split into chlorine gas (Cl2), sodium hydroxide (NaOH), and hydrogen gas (H2). The chlorine gas is collected separately for other uses, while the sodium hydroxide and hydrogen gas are the desired products.
3. Chlor Alkali Concentration Techniques
3.1 Purpose and Importance of Concentration
After the caustic is produced, it needs to be concentrated to increase its purity and strength. Concentration is essential as it allows for better control of chemical reactions and ensures the desired quality and concentration of caustic for various applications.
3.2 Vacuum Evaporation Technique
One common technique for caustic concentration is vacuum evaporation. This method involves heating the caustic solution under reduced pressure, which lowers the boiling point of water. As the water evaporates, the caustic solution becomes more concentrated. The evaporated water is then condensed and often recycled for further use.
3.3 Membrane Cell Process for Concentration
Another technique used for caustic concentration is the membrane cell process. In this method, the caustic solution is passed through ion-selective membranes, allowing for the separation of sodium ions from other impurities. The purified caustic solution is then concentrated further using vacuum evaporation or other methods, depending on the desired concentration level.
4. Flaking Process in Chlor Alkali Production
4.1 Role and Significance of Flaking
The flaking process is an important step in chlor alkali production, particularly for caustic. Flaking involves the solidification of liquid caustic into solid flakes for easy handling, storage, and transportation. It increases the surface area of caustic, facilitating its dissolution and incorporation into various manufacturing processes.
4.2 Flaking Equipment and Machinery
To achieve flaking, specialized flaking machines are used. These machines typically consist of rotating drums or pressing systems that solidify the liquid caustic and form it into flakes. The equipment ensures consistent and uniform flake size for optimal usability.
4.3 Flaking Process Steps
The flaking process typically involves cooling the liquid caustic and feeding it into the flaking machine. The machine uses a combination of pressure and cooling to solidify the caustic into flakes. The flakes are then collected, dried, and packaged for storage or transportation.
In conclusion, chlor alkali production is an essential industry that provides chlorine, caustic, and hydrogen gas. The production process involves electrolysis of brine to produce caustic, which is then concentrated through techniques such as vacuum evaporation or membrane cell processes. The flaking process further enhances the usability and convenience of caustic by transforming it into solid flakes. Understanding these processes helps us appreciate the significance of chlor alkali production and its ubiquitous presence in our daily lives.
5. Environmental and Safety Considerations in Chlor Alkali Production
5.1 Environmental Impacts and Regulations
When it comes to chlor alkali production, it’s not just about making those essential chemicals—it’s also crucial to consider the environmental impacts. The production process can have significant implications for the environment, particularly when it comes to waste management, air emissions, and water pollution.
One of the primary concerns in chlor alkali production is the management of mercury waste. Historically, mercury cells were used in the electrolysis process, leading to the release of mercury vapor and the accumulation of mercury waste. Thankfully, many facilities have now transitioned to mercury-free technologies, such as membrane cells, which have drastically reduced the amount of mercury released into the environment.
Stringent regulations and guidelines play a vital role in ensuring that chlor alkali production plants operate in an environmentally responsible manner. These regulations set limits on the release of pollutants and require proper treatment and disposal of waste materials. Compliance with these regulations helps minimize the environmental impact and protect ecosystems and local communities.
5.2 Safety Measures in Chlor Alkali Production
Safety is a top priority in any industrial process, and chlor alkali production is no exception. The production process involves handling hazardous substances and operating complex equipment, which requires strict adherence to safety protocols to prevent accidents and ensure the well-being of workers.
To manage the risks associated with chlor alkali production, facilities implement comprehensive safety measures. These measures include training programs to ensure employees understand the potential hazards and know how to safely handle chemicals and operate equipment. Personal protective equipment, such as gloves, goggles, and protective clothing, is also provided to mitigate exposure to hazardous substances.
In addition to personal safety, measures are in place to prevent and mitigate potential incidents, such as equipment failure, leaks, or spills. Regular maintenance and inspection of equipment, as well as emergency response plans and containment systems, are crucial in minimizing the impact of any unforeseen events.
By prioritizing safety and continuously improving safety systems, chlor alkali production facilities strive to create a work environment where employees can confidently carry out their tasks while maintaining their well-being.
Now that we’ve covered the environmental and safety considerations in chlor alkali production, let’s explore the fascinating process of producing caustic soda, from concentration to flaking.In conclusion, the chlor alkali production process, with its emphasis on caustic production, concentration techniques, and flaking process, is a crucial component of various industries. By delving into the details of these processes, we gain a deeper understanding of the intricacies involved in producing chlorine, sodium hydroxide, and hydrogen gas. Furthermore, it is essential to prioritize environmental sustainability and adhere to strict safety measures throughout the production journey. Through continuous advancements and adherence to best practices, the chlor alkali industry can continue to meet the growing demand for these essential chemicals while minimizing its ecological impact.
FAQs
1. What is the purpose of caustic soda in chlor alkali production?
Caustic soda, or sodium hydroxide, is a crucial product of the chlor alkali production process. It serves various purposes in industries such as chemical manufacturing, water treatment, and soap production. Caustic soda is used as a strong base, pH adjuster, and neutralizer. It is also a key ingredient in the production of detergents, paper, textiles, and various chemical compounds.
2. What are the concentration techniques employed in chlor alkali production?
There are several concentration techniques used in chlor alkali production to increase the concentration of sodium hydroxide. The most common methods include vacuum evaporation and the membrane cell process. Vacuum evaporation involves heating the solution to evaporate the water, leaving behind concentrated caustic soda. The membrane cell process uses ion-selective membranes to separate sodium hydroxide from other electrolysis products, resulting in concentrated caustic soda.
3. What is the purpose of the flaking process in chlor alkali production?
The flaking process is an essential step in chlor alkali production, specifically in the production of caustic soda. The purpose of flaking is to convert the liquid caustic soda into solid flakes or pellets. This solid form is easier to handle, transport, and store. Flakes or pellets of caustic soda have a high purity level and are widely used in various industries, including water treatment, chemical production, and alumina refining.
4. How does the chlor alkali production process prioritize environmental sustainability?
Environmental sustainability is a significant consideration in chlor alkali production. The industry has implemented various measures to minimize its ecological impact. These include the use of advanced technology and processes to reduce energy consumption, the implementation of efficient waste management systems, and the adoption of sustainable raw materials. Additionally, stringent environmental regulations and emission control mechanisms are in place to ensure compliance and minimize pollution associated with chlor alkali production.









