Introduction:
Caustic Soda Production and environment: Caustic soda, or sodium hydroxide (NaOH), is a fundamental chemical with widespread industrial applications. Its production involves the electrolysis of sodium chloride (NaCl) in a process known as the chlor-alkali process. While essential for various industries, the environmental impact of caustic soda production, particularly in the context of the electrolysis process, is a subject of growing concern. This article explores the environmental aspects of caustic soda production, focusing on the chlor-alkali electrolysis process and highlighting efforts to minimize its environmental footprint.

Chlor-Alkali Electrolysis Process: An Overview:
The chlor-alkali process, the predominant method for caustic soda production, involves the electrolysis of sodium chloride, resulting in the formation of caustic soda (NaOH) and chlorine gas (Cl2). The process typically occurs in mercury cells, membrane cells, or diaphragm cells. Each variant has distinct environmental implications.
Caustic Soda Production and environment
Mercury Cell Technology:
Historically, the mercury cell process was widely used. However, this method has significant environmental concerns due to the use of mercury. Mercury is a toxic heavy metal with detrimental effects on ecosystems and human health. Efforts have been made to phase out mercury cell technology in favor of more environmentally friendly alternatives.
Membrane Cell Technology:
The membrane cell process represents a more environmentally friendly alternative to mercury cell technology. It avoids the use of mercury, as it employs a selective membrane that allows the passage of sodium ions while preventing the migration of chloride ions and hydroxide ions. This reduces the environmental impact associated with mercury discharge.
Diaphragm Cell Technology:
The diaphragm cell process also eliminates the use of mercury. It involves a diaphragm that separates the anode and cathode compartments, preventing the direct mixing of chlorine and sodium hydroxide. While an improvement over mercury cell technology, diaphragm cell technology still has environmental considerations related to the disposal of the diaphragm material.
Environmental Considerations:
Mercury Contamination:
Historically, mercury cell technology was a major source of environmental pollution. Mercury contamination resulted from the use of mercury electrodes. Today, the phase-out of mercury cell technology has significantly reduced this environmental risk.
Chlorine Gas Emissions:
The production of chlorine gas as a byproduct poses environmental challenges. Chlorine is a highly reactive gas that, when released into the atmosphere, can contribute to air pollution. Modern technologies strive to capture and manage chlorine emissions to minimize their impact on air quality.
Energy Consumption:
The electrolysis process is energy-intensive. The production of caustic soda requires a substantial amount of electricity. The environmental impact is closely linked to the source of this electricity. Processes powered by renewable energy contribute less to greenhouse gas emissions compared to those relying on fossil fuels.
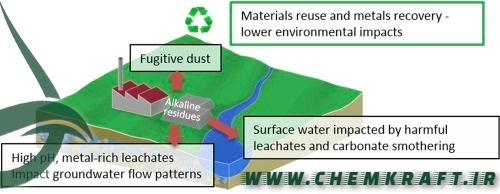
Brine Disposal:
The chlor-alkali process generates a brine solution as a byproduct. Disposing of this brine can have environmental consequences, especially if released directly into water bodies. Responsible disposal methods, such as brine treatment or injection into deep wells, aim to mitigate the impact on aquatic ecosystems.
Carbon Footprint:
The carbon footprint of caustic soda production is influenced by the energy source. If the electricity used in the electrolysis process comes from fossil fuels, the carbon emissions associated with electricity generation contribute to the overall environmental impact. Transitioning to renewable energy sources is a key strategy to reduce the carbon footprint.
Caustic Soda Production and environment
Environmental Mitigation Strategies:
Technology Upgrades:
Transitioning to more environmentally friendly technologies, such as membrane cell or diaphragm cell processes, helps reduce the environmental impact. These technologies eliminate the use of mercury and offer improved efficiency in chlorine and caustic soda production.
Renewable Energy Integration:
The environmental impact of caustic soda production can be mitigated by integrating renewable energy sources into the electrolysis process. Using electricity from solar, wind, or hydropower reduces the carbon footprint and contributes to sustainable production practices.
Efficient Resource Use:
Optimizing resource utilization, including minimizing raw material consumption and maximizing the efficiency of the electrolysis process, can reduce waste generation and environmental impact.
Brine Management:
Responsible brine management practices, such as brine treatment and deep-well injection, ensure that byproducts are handled in an environmentally sustainable manner, minimizing adverse effects on aquatic ecosystems.
Closed-Loop Systems:
Implementing closed-loop systems that recycle water and minimize discharges helps reduce the environmental footprint of caustic soda production. This approach contributes to water conservation and protects local water resources.
Conclusion:
The environmental considerations surrounding caustic soda production in the electrolysis process are multifaceted, involving issues such as mercury contamination, chlorine gas emissions, energy consumption, and brine disposal. While historical practices raised significant environmental concerns, technological advancements and industry awareness have driven improvements.
The transition away from mercury cell technology to more sustainable alternatives, coupled with the integration of renewable energy sources, represents a positive shift toward environmentally responsible caustic soda production. Ongoing efforts to optimize resource use, implement efficient technologies, and manage byproducts responsibly contribute to the industry’s commitment to environmental sustainability.
As the demand for caustic soda continues to grow, a proactive approach that prioritizes environmental responsibility is essential. By embracing innovative technologies and sustainable practices, the caustic soda industry can strike a balance between meeting global demand and minimizing its environmental impact. This commitment not only aligns with corporate social responsibility but also ensures a sustainable and resilient future for the industry and the ecosystems it interacts with.