| H2ZrF6 |
| Hexafluorozirconic Acid |
| Corrosive liquid |
| CAS No.: 12021-95-5 EU No.: 234-666-0 UN No.: 2922 |
Introduction
Hexafluorozirconic acid, also known as H2ZrF6, is a highly corrosive and versatile chemical compound with various industrial applications. In this essay, we will explore the characteristics and applications of fluorozirconic acid.
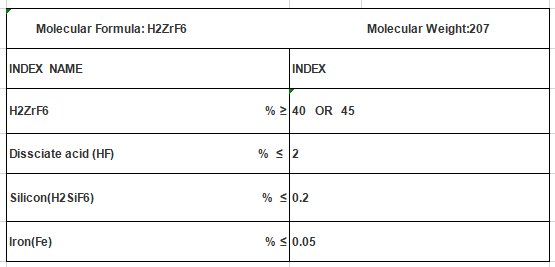
In terms of its characteristics, hexafluorozirconic acid is a colorless, odorless liquid with a molecular weight of 207.214 g/mol. It is highly soluble in water and exhibits a strong acidic nature due to its ability to dissociate into hydrogen ions and zirconium hexafluoride ions. This acid is known for its stability at high temperatures, enabling its use in various manufacturing processes. Furthermore, it has a strong oxidizing capability, making it a valuable compound for certain chemical reactions.
Applications
One of the significant applications of fluorozirconic acid is in the production of electroplating solutions. With its strong oxidizing properties, it serves as an effective oxidizer in the preparation of gold plating baths. This acid facilitates the formation of a thin layer of zirconium oxide on the surface, which enhances the adhesion and durability of the gold coating. Moreover, fluorozirconic acid is utilized in the synthesis of other metal plating solutions, such as nickel and chromium, for various industrial applications.
Another notable application
is in the production of ceramic materials. It is commonly employed as a precursor for doping zirconia powders to enhance their electrical and thermal conductivity. By incorporating fluorozirconic acid into the manufacturing process, the properties of the ceramics can be tailored to meet specific requirements. This acid acts as a catalyst during the synthesis, resulting in improved sintering and densification of the ceramic material.
Furthermore, this compound finds application in the textile industry. It is used as a stabilizer in the printing of textiles, particularly for reactive dyes. The acid helps to control the pH levels during the printing process, ensuring optimum fixation of dyes onto the fabric. Additionally, hexafluorozirconic acid acts as an anti-septic and anti-microbial agent, preventing the growth of bacteria or fungi on textile surfaces. This property makes it an ideal additive in the production of antimicrobial textiles, such as hospital linens or sportswear.
Other applications
Lastly, this acid has applications in the production of specialty chemicals. It is commonly used as a fluorinating agent in organic synthesis, enabling the introduction of fluorine atoms into various compounds. This chemical transformation can impart desirable properties to the synthesized compounds, such as increased stability or improved solubility. Furthermore, hexafluorozirconic acid can be employed in the synthesis of pharmaceutical intermediates, agricultural chemicals, and other fine chemicals, contributing to the development of advanced materials and technologies.
In conclusion
hexafluorozirconic acid is a highly corrosive and versatile compound with numerous industrial applications. Its stability, oxidizing capability, and reactive nature make it a valuable component in electroplating solutions, ceramic materials, textile printing, and specialty chemical synthesis. As research and technology continue to advance, it is likely that further applications for hexafluorozirconic acid will be discovered, further expanding its importance in various industries.
Applications of fluorozirconic acid:
Fluorozirconic Acid, also known as hexafluorozirconic acid, is a powerful and versatile chemical compound with a wide range of applications across various industries. Its unique properties and reactivity make it a valuable component in numerous processes, contributing to advancements in technology, manufacturing, and research. This essay explores the diverse applications of Fluorozirconic Acid, shedding light on its importance and impact on different sectors.
Chemical Properties:
Fluorozirconic Acid (H2ZrF6) is derived from the reaction between zirconium dioxide (ZrO2) and hydrofluoric acid (HF). This results in a highly acidic and corrosive solution. Its exceptional chemical stability, strong acidity, and solubility in water are key attributes that contribute to its widespread applications.
Metal Surface Treatment:
Fluorozirconic Acid finds extensive use in metal surface treatment processes, particularly in the preparation of aluminum surfaces for anodizing. When applied as an etchant, it helps remove impurities and oxides from the metal surface, enhancing adhesion and promoting uniform anodized coatings. The treated metal surfaces exhibit improved corrosion resistance and durability, making them suitable for various applications, such as aerospace components and architectural finishes.
Electroplating:
In the field of electroplating, Fluorozirconic Acid plays a crucial role in enhancing the quality and efficiency of plating processes. It is used as an additive to electroplating baths for metals like nickel and zinc. This results in a smoother and more uniform deposition of metal, leading to improved corrosion resistance and durability of the plated surfaces. The application of Fluorozirconic Acid in electroplating contributes to the production of high-quality coated products used in electronics, automotive, and decorative industries.
Catalysts in Organic Synthesis:
Fluorozirconic Acid serves as a strong Lewis acid catalyst in various organic synthesis reactions. Its ability to activate certain substrates and facilitate reactions such as Friedel-Crafts acylation and alkylation makes it a valuable tool in the production of pharmaceuticals, specialty chemicals, and polymers. The use of Fluorozirconic Acid as a catalyst allows for more efficient and selective reactions, reducing the need for harsher reaction conditions and improving overall process sustainability.
Glass Etching:
Fluorozirconic Acid is employed in the glass industry for its ability to etch glass surfaces. The acid selectively removes thin layers of glass, creating intricate designs or patterns. This application is particularly useful in the production of decorative glassware, glass art, and electronic components. The controlled etching process enabled by Fluorozirconic Acid allows for precise and intricate designs, adding aesthetic value to glass products.
Uranium Extraction:
In the nuclear industry, Fluorozirconic Acid plays a vital role in the extraction and purification of uranium. It is used to convert raw uranium ore into a form suitable for further processing. The acid reacts with uranium compounds to form soluble complexes, facilitating the separation and extraction of uranium. This application is essential for the production of nuclear fuel and plays a crucial role in the generation of nuclear energy.
Fluoride Production:
Fluorozirconic Acid is a significant source of fluoride ions, and its decomposition releases fluoride. This property makes it valuable in the production of fluorides used in various industrial processes. Sodium fluoride, potassium fluoride, and other fluoride salts can be derived from Fluorozirconic Acid, finding applications in water fluoridation, metal surface treatment, and the manufacturing of specialized chemicals.
Conclusion:
In conclusion, Fluorozirconic Acid stands as a versatile and indispensable chemical with diverse applications across different industries. Its unique chemical properties, including strong acidity, stability, and solubility, make it valuable in metal surface treatment, electroplating, organic synthesis, glass etching, uranium extraction, and fluoride production. The widespread use of Fluorozirconic Acid highlights its pivotal role in advancing technology, improving manufacturing processes, and contributing to the development of various industrial sectors. As research continues, it is likely that new applications for this compound will be discovered, further expanding its role in shaping the future of chemistry and industry.



