Hexafluorotitanic acid
Hexafluorotitanic acid, also known as titanium hexafluoride, is a highly important chemical compound in various industries. This acid has several synonyms, including hydrofluorotitanic acid and hydrofluorotitanic(IV) acid. Its chemical formula is H2TiF6, indicating two hydrogen atoms, one titanium atom, and six fluoride atoms bonded together. Hexafluorotitanic acid is widely utilized in diverse applications, including metal surface treatment, glass manufacturing, and as a catalyst in chemical reactions. Its CAS number is 17439-11-1, which serves as an identification code within chemical databases and labeling systems.
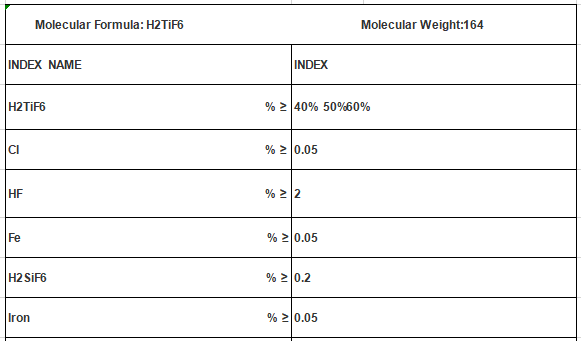
Applications
This acid finds extensive utility in metal surface treatment processes. It is commonly used as an etchant or cleaning agent for metals, such as titanium and its alloys. The acid’s corrosive properties enable it to remove oxides, contaminants, and unwanted materials, resulting in improved metal surfaces for further treatments or applications. Additionally, hexafluorotitanic acid helps to enhance the corrosion resistance and adhesion of metals, making it a crucial component in industries like aerospace, automotive, and electronics.
Other applications
Another significant application of hexafluorotitanic acid lies in the glass manufacturing industry. This compound plays a vital role in the production of high-quality glass by acting as a flux. Hexafluorotitanic acid helps to lower the melting point of the glass batch, allowing for easier and more efficient glass formation during the manufacturing process. Furthermore, it imparts desirable properties to the glass, such as increased refractive index and resistance to chemical attack. As a result, hexafluorotitanic acid finds extensive use in the production of optical lenses, glass fibers, and various specialty glasses.
Hexafluorotitanic acid also acts as a catalyst in numerous chemical reactions. Its acidic nature and strong oxidizing properties make it an effective catalyst for several organic and inorganic reactions. Specifically, it facilitates the production of various organic compounds, such as aldehydes, ketones, and amines, through processes like oxidation and rearrangement reactions. In addition, hexafluorotitanic acid is utilized in the synthesis of inorganic compounds, including fluorides and titanium-containing substances. Its catalytic versatility and efficiency make it a valuable asset in the pharmaceutical, chemical, and research sectors.
Conclusion
In conclusion, hexafluorotitanic acid, with its synonyms of hydrofluorotitanic acid and hydrofluorotitanic(IV) acid, offers a wide array of applications across various industries. Its effective metal surface treatment properties, usage as a flux in glass manufacturing, and catalytic capabilities in chemical reactions contribute to its significance. The CAS number 17439-11-1 serves as a unique identifier for this compound within chemical databases. As technology advances, the utilization and demand for this kind of acid is likely to continue growing, as it remains an invaluable component in numerous industrial processes.
Applications of Hexafluorotitanic Acid: A Comprehensive Overview
Introduction:
Hexafluorotitanic Acid, with the chemical formula H2TiF6, is a notable compound that plays a crucial role in various industrial applications. Formed through the reaction of titanium dioxide with hydrofluoric acid, this colorless and corrosive liquid exhibits unique properties that make it valuable in different sectors. This essay explores the diverse applications of Hexafluorotitanic Acid, shedding light on its significance and contributions to various industries.
Chemical Properties:
Hexafluorotitanic Acid is characterized by its strong acidity and solubility in water. It contains titanium in its highest oxidation state, providing it with unique reactivity and stability. These properties make Hexafluorotitanic Acid an important component in a range of industrial processes.
Applications:
- Electroplating: Hexafluorotitanic Acid is widely used in the electroplating industry. It serves as an electrolyte in the deposition of titanium-based coatings on various substrates. The electroplating process with Hexafluorotitanic Acid enhances the corrosion resistance and durability of surfaces, making it particularly valuable in industries such as aerospace, automotive, and electronics.
- Surface Treatment and Cleaning: In metal treatment and cleaning processes, Hexafluorotitanic Acid is employed to remove oxide layers and impurities from metal surfaces. This application is crucial in preparing surfaces for subsequent treatments like coating, painting, or bonding. The use of Hexafluorotitanic Acid ensures the formation of clean and reactive metal surfaces, enhancing the overall quality of finished products.
- Catalyst in Organic Synthesis: Hexafluorotitanic Acid acts as a Lewis acid catalyst in various organic synthesis reactions. Its catalytic properties facilitate the transformation of organic compounds, enabling the production of specialty chemicals, pharmaceuticals, and polymers. The use of Hexafluorotitanic Acid in catalysis contributes to more sustainable and efficient synthetic processes.
- Titanium Derivatives Production: Hexafluorotitanic Acid serves as a precursor for the production of various titanium derivatives. Through controlled reactions, it can be converted into compounds used in the manufacturing of pigments, catalysts, and titanium-containing materials. These derivatives find applications in industries ranging from paints and coatings to aerospace technologies.
- Etching Agent in Electronics: In the electronics industry, Hexafluorotitanic Acid is utilized as an etching agent. It selectively removes oxides from the surfaces of semiconductor materials, allowing for the precise patterning of electronic components. This application is essential in the manufacturing of microelectronics and integrated circuits.
- Photography and Printing: Hexafluorotitanic Acid finds application in the field of photography and printing. It is used in certain processes to prepare aluminum plates for lithographic printing. The acid facilitates the formation of a hydrophilic surface on the aluminum plates, ensuring accurate and high-quality printing.
Controlling Organizations:
The production, handling, and use of fluorine compounds, including Hexafluorotitanic Acid, are subject to regulations and guidelines set by various international, national, and regional bodies. One prominent organization overseeing the safety and regulation of chemicals is the International Council of Chemical Associations (ICCA). The ICCA collaborates with industry stakeholders, governments, and other organizations to promote the responsible use of chemicals, including fluorine compounds. More specific regulations may also be in place depending on the region or country, often enforced by environmental agencies and regulatory bodies.
For detailed information on regulations and safety guidelines related to Hexafluorotitanic Acid and other fluorine compounds, individuals and organizations can refer to the websites of regulatory bodies such as the Environmental Protection Agency (EPA) in the United States or the European Chemicals Agency (ECHA) in Europe. These agencies provide comprehensive information on the safe handling, storage, and disposal of chemicals, contributing to the responsible and sustainable use of compounds like Hexafluorotitanic Acid.


