Definition
Hexafluorosilicic acid is a chemical compound that is widely used in various industries and has numerous applications. This essay will provide an overview of this acid, including its analysis, formula, and applications.
Hexafluorosilicic acid, also known as hydrofluorosilicic acid, is an inorganic compound with the chemical formula H2SiF6. The acid is derived from silicon fluoride, and it is a colorless liquid with a strong, pungent odor. In terms of its analysis, hexafluorosilicic acid can be identified through various laboratory techniques such as spectroscopy and chromatography.
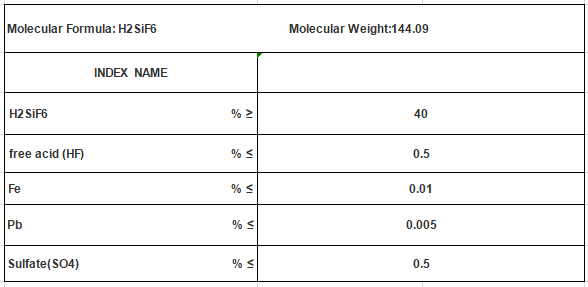
Analysis
Chemical formula of hexafluorosilicic acid indicates that it consists of two hydrogen (H) atoms, one silicon (Si) atom, and six fluorine (F) atoms. Presence of silicon in the formula highlights its connection to the silicon family of elements. The six fluorine atoms indicate that this acid is highly reactive and capable of forming strong bonds with other elements and compounds.
Applications
Hexafluorosilicic acid has numerous applications across different industries. One of its most common uses is in water fluoridation. It is added to drinking water supplies to prevent tooth decay and improve dental health. The acid reacts with the enamel of the teeth, forming a protective layer that makes them resistant to acids produced by bacteria. This application has been widely adopted and has significantly contributed to overall dental health.
Another important application of hexafluorosilicic acid is in the production of aluminum. It is used as a flux in aluminum smelting processes to remove impurities and improve the quality of the final product. Additionally, hexafluorosilicic acid is utilized in the manufacturing of synthetic cryolite, which is an important component in the production of aluminium.
In the chemical industry, hexafluorosilicic acid is used for various purposes including the synthesis of other chemical compounds such as organo-fluoro compounds and pharmaceuticals. It is also employed as a catalyst in certain organic reactions and as an etching agent for glass and ceramic surfaces.
However, it is important to note that the use of hexafluorosilicic acid should be handled with care due to its toxic and acidic nature. Proper precautions and safety measures should be taken to ensure its safe handling and storage.
Conclusion
In conclusion, hexafluorosilicic acid is a versatile and useful chemical compound with various applications. Its analysis can be carried out through laboratory techniques, and its chemical formula indicates its composition and reactivity. From water fluoridation to the production of aluminum and pharmaceutical synthesis, this acid plays a significant role in multiple industries. Nonetheless, caution should be exercised when working with this acid due to its toxicity.
Applications:
Hexafluorosilicic Acid, often denoted as H2SiF6, is a chemical compound derived from the reaction between silicon dioxide and hydrofluoric acid. This colorless, corrosive liquid exhibits unique properties that make it a valuable component in various industrial processes. This essay delves into the diverse applications of Hexafluorosilicic Acid, exploring its significance and contributions across different sectors.
Chemical Properties:
Hexafluorosilicic Acid is characterized by its strong acidity and remarkable solubility in water. It is produced as a byproduct in the production of phosphate fertilizers, making it an economically viable raw material for several applications. Its stability and reactivity contribute to its widespread use in diverse industries.
Water Fluoridation:
One of the most common applications of Hexafluorosilicic Acid is in water fluoridation programs. When added to public water supplies, it releases fluoride ions, helping prevent tooth decay and promoting dental health. This practice is widely adopted worldwide as a cost-effective and efficient means of providing a controlled and consistent fluoride concentration in drinking water. Hexafluorosilicic Acid’s role in water fluoridation has played a crucial role in improving public dental health over the years.
Metal Surface Treatment:
Hexafluorosilicic Acid is employed in the metal industry for its role in surface treatment processes. It acts as an effective pickling agent, removing oxides and impurities from metal surfaces before further processing. This application is crucial in preparing metal substrates for coating, plating, or other treatments, ensuring enhanced adhesion and improved corrosion resistance. Industries such as automotive, aerospace, and electronics benefit from the use of Hexafluorosilicic Acid in metal surface treatment.
Aluminum Production:
In the aluminum industry, Hexafluorosilicic Acid plays a role in the production of aluminum metal. It is used during the refining process of bauxite ore to extract aluminum oxide, which is then electrolyzed to obtain aluminum. The addition of Hexafluorosilicic Acid helps remove impurities and contributes to the overall efficiency of the aluminum production process.
Catalyst in Chemical Synthesis:
Hexafluorosilicic Acid serves as a catalyst in various chemical synthesis reactions. Its Lewis acidic properties make it suitable for promoting certain organic transformations, such as Friedel-Crafts alkylation and acylation reactions. The use of Hexafluorosilicic Acid as a catalyst enhances reaction rates and selectivity, contributing to the efficient synthesis of pharmaceuticals, agrochemicals, and specialty chemicals.
Concrete and Cement Additive:
Hexafluorosilicic Acid finds application as an additive in the production of concrete and cement. It reacts with alkaline compounds in cement to form compounds that enhance the strength and durability of concrete structures. This application is particularly significant in construction, as it contributes to the development of long-lasting and resilient infrastructure.
Fluoride Derivatives Production:
Hexafluorosilicic Acid serves as a precursor for the production of various fluoride derivatives. Through controlled reactions and processing, it can be converted into different fluoride compounds used in diverse applications. Sodium hexafluorosilicate and potassium hexafluorosilicate are examples of derivatives that find use in manufacturing, water treatment, and other industrial processes.
Oil and Gas Industry:
In the oil and gas industry, Hexafluorosilicic Acid is employed in well stimulation processes. It helps dissolve minerals and improve the permeability of rocks, enhancing the extraction of oil and gas from reservoirs. This application contributes to the efficiency of hydrocarbon extraction operations.
Conclusion:
Hexafluorosilicic Acid emerges as a multifaceted chemical compound with a broad spectrum of applications across various industries. Its role in water fluoridation, metal surface treatment, aluminum production, chemical synthesis, concrete additives, fluoride derivatives production, and the oil and gas industry underscores its versatility and importance. As technology advances and research continues, Hexafluorosilicic Acid is likely to find new applications, further solidifying its position as a valuable component in industrial processes. The compound’s ability to address diverse needs across different sectors highlights its impact on improving efficiency, sustainability, and overall industrial development.


