Information
Potassium bifluoride, also known as potassium hydrogen fluoride, is a valuable compound with various production methods and applications in different industries. This essay will explore the process of producing potassium bifluoride and discuss its wide range of applications.
Potassium Bifluoride KHF2 CAS NO. : 7789-29-9
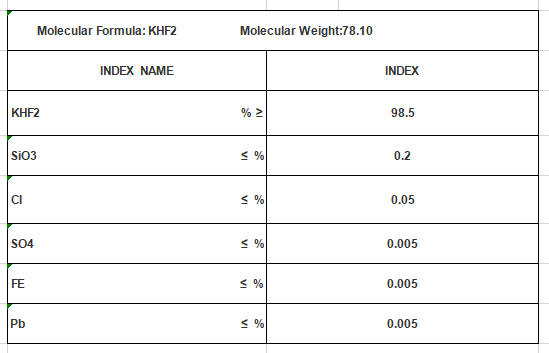
Molecular Formula: KHF2
Mol. Wt: 78.10
Cas No.: 7789-29-9
UN No.: 1811
EMS No.: F-A,S-B
EINECS: 232-156-2
Synonyms:Fluorine potassium hydride,Potassium Fluoride,potassium acid fluoride
Physical state: White to light gray crystal or crystalline powder
Relative density: 2.37 g/mL at 25 °C(lit.)
Melting point: 239 °C(lit.)
Solubility: 39 g/100 mL (20 ºC)
Regular package: 25 kg / bag, or according to customer’s requirements
Storage: Hygroscopic. Keep container tightly closed in a cool, dry and well-ventilated place
Transportation: Hazard Chemical, Hazard Class: 8, Packing
Group: II, UN No.: 1811
The production of potassium bifluoride involves the reaction between hydrogen fluoride and potassium hydroxide or potassium carbonate. This reaction results in the formation of potassium bifluoride and water. The process typically takes place in a specialized reactor where the reactants are carefully controlled and monitored. The purity of the final product depends on the quality of the starting materials and the precise conditions of the reaction. To ensure high purity, industrial production may involve multiple purification steps, such as filtration and distillation.
Applications
Potassium bifluoride finds applications in various industries due to its unique properties. One of its primary uses is in aluminum smelting and refining. Potassium bifluoride acts as a fluxing agent, helping to remove impurities and lower the melting point of aluminum oxide during the smelting process. This compound is also utilized in the production of synthetic cryolite, which is an essential component in aluminum electrolysis cells. Its ability to dissolve aluminum oxide makes it an indispensable compound in the aluminum industry.
Main application
Another significant application of potassium bifluoride is in the glass industry. It is used as a glass etchant or surface cleaning agent due to its strong etching properties. Potassium bifluoride can remove unwanted residues and contaminants from glass surfaces, ensuring high-quality finished products. Additionally, it can act as a welding flux in the manufacturing of optical lenses, further demonstrating its versatility in the glass sector.
Production of fluorine compounds
Furthermore, potassium bifluoride is employed in the production of fluorine compounds, such as fluorides and fluorosilicates. These compounds find applications in various fields, including manufacturing pharmaceuticals, pesticides, and dyes. Potassium bifluoride’s ability to release fluorine ions enables it to participate in a wide range of chemical reactions, leading to the synthesis of valuable compounds that are essential in many industries.
In addition to its industrial applications, potassium bifluoride also has various uses in research and development. It is commonly employed as a catalyst or reactant in laboratory experiments and chemical synthesis. The compound’s unique properties, such as its acidity and solubility, make it valuable for studying and understanding various chemical reactions. Its widespread usage in research laboratories underscores its significance in scientific advancements.
Conclusion
In conclusion, potassium bifluoride is a compound produced through the reaction between hydrogen fluoride and potassium hydroxide or potassium carbonate. Its production involves precise control and purification steps to ensure high purity. The compound finds applications in the aluminum and glass industries, as well as the production of fluorine compounds. Its ability to act as a fluxing agent, glass etchant, and catalyst makes it indispensable in various fields. Furthermore, its use in research and development contributes to scientific advancement and understanding. This chemical is truly a versatile compound with numerous important applications.


