Information
Sodium Tetrafluoroborate, Quality CAS NO. 13755-29-8, Sodium fluoroborate
CAS NO: 13755-29-
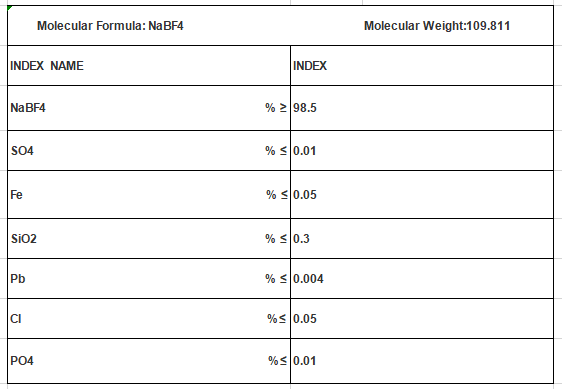
Molecular Formula: NaBF4
Mol. Wt: 109.811
Cas No.: 13755-29-8
EINECS: 237-531-4
Synonyms: Sodium Tetrafluoroborate, Sodium Fluoride, Sodium Acid Fluoride
Physical state: White or colorless crystal
Relative density: 2.47g/mL, at 25°C(lit.)
Melting point: 384°C
Stability: Moisture sensitive; do not store in glass (though the completely dry material does not etch glass). Incompatible with strong oxidizing agents
Package: 25kg/bag, or according to customer’s requirements

Storage: Moisture sensitive. Keep container tightly closed in a dry and well-ventilated place. Handle and store under inert gas
Transportation: Not dangerous good, but moisture sensitive, should keep it dry
Sodium fluoroborate, also known as sodium tetrafluoroborate, is a chemical compound with the formula NaBF4. It is a white crystalline powder that is highly soluble in water. Sodium fluoroborate is produced through a reaction between sodium borofluoride and sodium hydroxide, resulting in the formation of this chemical and water.
The production of sodium fluoroborate involves a two-step process. Firstly, sodium hydroxide (NaOH) is mixed with hydrofluoric acid (HF) to form sodium borofluoride (NaBF4). This reaction involves the exchange of the hydroxide ion (OH-) in sodium hydroxide with the fluoride ion (F-) from hydrofluoric acid. In the second step, sodium borofluoride is then reacted with sodium hydroxide again, resulting in the production of sodium fluoroborate (NaBF4) and water (H2O). The reaction is as follows:
NaBF4 + NaOH → NaBF4 + H2O
Sodium fluoroborate has numerous applications in various industries. One of its primary uses is as a source of fluoride ions in electroplating processes. It is commonly used in the plating of metals such as tin, zinc, and nickel. This compound acts as a fluxing agent, helping to remove impurities and improve the quality of the plated metal.
Applicatuins
Another significant application of this chemical is in the production of soldering fluxes. Fluxes are used to facilitate soldering by preventing oxidation of the metal surfaces being joined. Sodium fluoroborate-containing fluxes are particularly effective in soldering aluminum and aluminum alloys, as they form a protective layer on the metal surface, preventing the formation of oxide layers.
Sodium fluoroborate is also utilized in the manufacturing of specialized glasses, ceramics, and glazes. Its addition to these materials lowers their melting points and improves their viscosity, making them easier to work with. Moreover, it enhances the chemical resistance and durability of the resulting products. Additionally, due to its high solubility in water, it can be used as an electrolyte in batteries and fuel cells.
In summary
sodium fluoroborate is a vital chemical compound with diverse applications in various industries. Its production involves a reaction between sodium borofluoride and sodium hydroxide. It is used as a source of fluoride ions in electroplating, soldering fluxes, and in the manufacturing of glasses, ceramics, and glazes. Sodium fluoroborate plays a crucial role in improving the quality, durability, and chemical resistance of various products.


