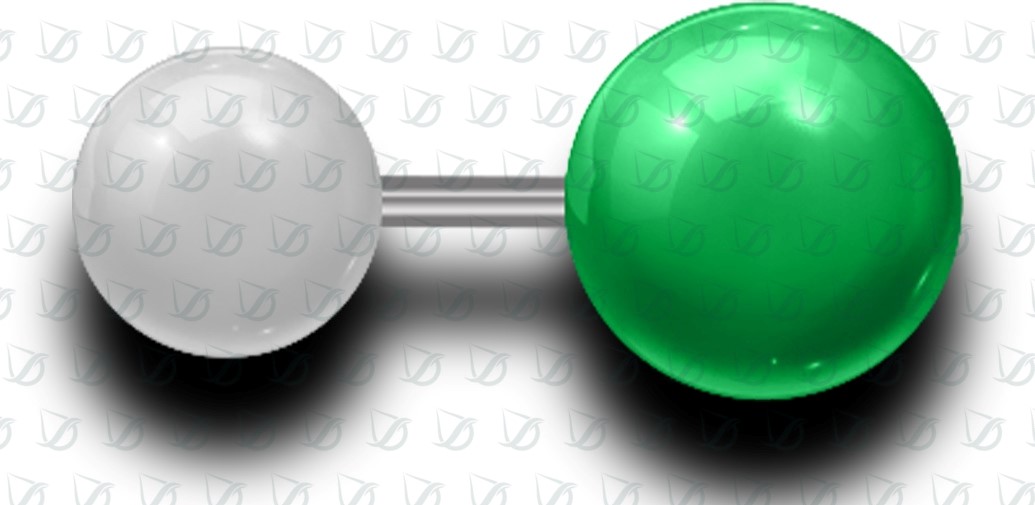
(HF)Introduction to Hydrofluoric Acid
Hydrofluoric acid, commonly abbreviated as HF, is a highly corrosive and toxic chemical compound. It is composed of hydrogen fluoride (HF) dissolved in water, forming a colorless liquid with a sour odor. Hydrofluoric acid is widely used in various industrial processes, including glass etching, petroleum refining, aluminum production, and chemical synthesis. However, due to its hazardous nature, the handling and management of hydrofluoric acid require strict safety precautions to protect both human health and the environment. This article provides an overview of hydrofluoric acid production, its applications in different industries, essential safety measures, proper handling techniques, emergency response procedures, and environmental considerations for its use and disposal.
What is Hydrofluoric Acid
Hydrofluoric acid, also known as HF, is a highly corrosive and dangerous acid that consists of hydrogen fluoride dissolved in water. It is a colorless liquid with a pungent odor and is considered one of the most aggressive acids.
Properties of Hydrofluoric Acid
HF acid has unique properties that make it both useful and hazardous. It has a high acidity and can react strongly with various materials including glass, metals, and organic compounds. It is also notable for its ability to dissolve silica, which is why it is commonly used for glass etching.
Common Uses of Hydrofluoric Acid
Hydrofluoric acid finds applications in several industries. It is widely used in the production of aluminum, petroleum refining, and in the synthesis of pharmaceuticals and various chemicals. Additionally, it is used for cleaning metal surfaces and etching glass due to its corrosive properties.
Production Methods of Hydrofluoric Acid
Direct Fluorination Process
In the direct fluorination process, HF acid is produced by reacting fluorine gas with various precursors such as sulfuric acid or liquid anhydrous hydrogen fluoride. This method is widely used on an industrial scale to obtain high purity hydrofluoric acid.
Fluorosilicic Acid Process
The fluorosilicic acid process involves treating fluorspar or calcium fluoride with sulfuric acid to produce hydrofluoric acid. This process is commonly used in smaller-scale production facilities.
Hydrolysis Process
The hydrolysis process is another method used to produce HF acid. It involves the reaction of anhydrous hydrogen fluoride with water, resulting in the formation of hydrofluoric acid. This process is less commonly used compared to the direct fluorination process.
Industrial Applications of Hydrofluoric Acid
Glass etching and Cleaning
One of the most well-known uses of HF acid is in glass etching and cleaning. Due to its ability to dissolve silica, it is used to create intricate designs on glass surfaces and remove impurities from glassware.
Petroleum Refining
Hydrofluoric acid plays a crucial role in petroleum refining processes. It is used to remove impurities and improve the quality of several petroleum products, such as gasoline and diesel fuel.
Aluminum Production
In the aluminum industry, hydrofluoric acid is used to remove oxide coatings from the surface of aluminum. This process, known as aluminum pickling, results in a cleaner and smoother finish.
Pharmaceuticals and Chemical Synthesis
HF acid is employed in the synthesis of various pharmaceuticals, including antibiotics and antiviral drugs. It is also used as a catalyst in several chemical reactions, contributing to the production of numerous chemicals and compounds.
Safety Precautions for Handling Hydrofluoric Acid
Understanding the Hazards of Hydrofluoric Acid
It is important to be aware of the hazards associated with HF acid. It can cause severe burns and tissue damage upon contact, both externally and internally. Inhalation of fumes or mist can lead to respiratory issues, while ingestion can be fatal.
Risk Assessment and Management
When handling hydrofluoric acid, a thorough risk assessment is essential. This involves identifying potential hazards, implementing safety protocols, and providing appropriate training to personnel. It is crucial to have emergency response plans in place and to ensure the availability of safety equipment, such as personal protective gear.
Engineering Controls and Ventilation
To minimize exposure to hydrofluoric acid, engineering controls should be implemented. This includes the use of fume hoods and ventilation systems to prevent the buildup of hazardous fumes and ensure a safe working environment. Regular monitoring of air quality is also recommended.
Remember, when it comes to handling HF acid, safety should always be a top priority. Take the necessary precautions, educate yourself, and follow proper procedures to avoid any accidents or injuries. Stay safe and handle with care!
Personal Protective Equipment (PPE) for Handling Hydrofluoric Acid
Selection and Proper Use of PPE
When it comes to handling HF acid, you definitely don’t want any “acid accidents” ruining your day. That’s where personal protective equipment (PPE) comes in. Picking the right gear and using it properly can make all the difference in keeping you safe and sound.
The selection of PPE should depend on the specific tasks you’re performing and the potential risks involved. Make sure to choose PPE that covers all exposed body parts, including your eyes, face, hands, and body. It’s like dressing up for a not-so-fashionable acid-themed party, but trust me, safety is always in style.
Recommended PPE for Different Scenarios
Now, let’s break down the recommended PPE for different acid-related scenarios.
– Eye and Face Protection: Wear chemical goggles or a full-face shield to shield your precious peepers from corrosive acid splashes. The last thing you want is a surprise acid attack ruining your vision for life.
– Gloves: Opt for gloves made from resistant materials like neoprene, nitrile, or butyl rubber. These superheroes will protect your hands from the acid’s harmful effects. Just remember, gloves can be your best friends, so treat them well and don’t leave them hanging.
– Protective Clothing: Wear acid-resistant lab coats or coveralls to keep your delicate skin safe. Pretend you’re a scientist in a top-secret lab, but without the explosions (hopefully).
– Footwear: Acid-resistant boots, like those made from rubber, are your go-to footwear choice. Keep your toes protected, because nobody wants a pedicure from hydrofluoric acid.
Remember, PPE is your armor against acid mishaps. Dress to protect, my friend!
Emergency Response and First Aid Procedures for HF Acid Exposure
Recognizing Hydrofluoric Acid Exposure
Picture this: you’re minding your own business, handling hydrofluoric acid like a pro, when suddenly, disaster strikes. How do you know if you’ve been exposed? Look out for these signs:
– Skin Contact: If the acid comes into contact with your skin, you might experience redness, pain, and potential burns. It’s like a bad date that leaves a lasting impression, but way less romantic.
– Eye Contact: Acid splashes can cause eye irritation, redness, or even severe damage. So, if your eyes start protesting with a fiery passion, it’s a clear sign something went wrong.
– Inhalation: Breathing in HF acid fumes can irritate your respiratory system. If you suddenly feel like you’ve entered a toxic gas chamber, that’s a sign to get out and seek help.
Immediate Actions in Case of Exposure
It’s important to act quickly if you find yourself in an “acid-entangled” situation. Follow these steps:
– Remove Contaminated Clothing: Strip away any clothing that has come into contact with the acid. Think of it as a “wardrobe malfunction” that requires an immediate fashion change.
– Rinse, Rinse, Rinse: Flush the affected area with copious amounts of water for at least 15 minutes. Remember, water is your acid-fighting ally, so let it do its job.
– Seek Medical Attention: Don’t be a hero in this situation. Even if your burns seem minor, seek medical help. Acid is nothing to mess around with.
First Aid Treatment for Hydrofluoric Acid Burns
If you or someone you know has fallen victim to HF acid burns, here’s how you can provide some first aid relief:
– Continue Flushing: Keep rinsing the affected area with cool water until medical help arrives. Think of it as your personal acid-based “shower time” without the luxury of singing in the rain.
– Don’t Apply Creams or Ointments: Avoid using any creams, ointments, or home remedies on the burns. Let the healthcare professionals handle the situation.
– Support and Comfort: Offer support and comfort to the affected person while waiting for medical assistance. A little empathy can go a long way in times of acid-related distress.
Stay safe and keep calm, even in the face of acid-induced chaos!
Handling and Storage Recommendations for HF Acid
Safe Handling Practices
Handling HF acid requires a delicate touch, much like dealing with a fragile friendship. Here are some safe handling practices to keep in mind:
– Ventilation: Always work in well-ventilated areas to prevent the buildup of acid fumes. Remember, you deserve to breathe fresh air, not corroding chemicals.
– Contain the Acid: Keep hydrofluoric acid in appropriate containers to avoid spills and leaks. Trust us, you don’t want an acid river flowing through your workspace.
– Avoid Mixing: Never mix hydrofluoric acid with other chemicals unless specifically instructed to do so. Acid mixology may be exciting at a bar, but in the lab, it’s a risky move.
– Careful Pouring: When transferring hydrofluoric acid, pour it slowly and carefully to avoid splashing or spilling. Think of it as pouring tea for the Queen, just with a lot more danger involved.
Storage Requirements and Guidelines
When it comes to storing HF acid, you need to treat it like a high-maintenance pet. Here are some guidelines to keep in mind:
– Acid-Proof Containers: Store hydrofluoric acid in appropriate acid-resistant containers. It’s like having a luxurious “acid-proof” hotel suite for your chemical companion.
– Cool and Dry: Keep the acid stored in a cool, dry place to maintain its stability. No one likes a hot-headed acid on the loose.
– Separate and Label: Store HF acid away from incompatible substances and make sure to label everything clearly. Nobody wants a chemistry mix-up that ends in disaster.
By following proper handling and storage practices, you can keep HF acid in line and avoid any chemical catastrophes.
Environmental Considerations and Disposal of Hydrofluoric Acid
Environmental Impact of HF Acid
Hydrofluoric acid isn’t just a danger to humans; it can also wreak havoc on the environment. Here are a few things to consider:
– Water Pollution: If hydrofluoric acid finds its way into water bodies, it can contaminate and harm aquatic life. Remember, fish don’t enjoy acid baths as much as you might think.
– Soil Contamination: Acid spills or leaks can lead to soil contamination, affecting plants and potentially leaching into groundwater. Acid-induced greenery doesn’t quite have the same appeal as a lush garden.
Proper Disposal Methods
When it’s time to bid farewell to hydrofluoric acid, make sure to follow these proper disposal methods:
– Neutralization
In conclusion, HF acid is a valuable chemical compound with diverse industrial applications. However, its corrosive and toxic nature demands utmost caution and adherence to safety protocols during handling and storage. By understanding the hazards, using appropriate personal protective equipment, and implementing proper safety measures, the risks associated with HF acid can be effectively managed. It is crucial to prioritize the protection of workers, the environment, and the surrounding communities. By following recommended guidelines and regulations, we can ensure the responsible and safe utilization of hydrofluoric acid in various industries while minimizing potential risks.
FAQ
?Is hydrofluoric acid dangerous to handle
Yes, hydrofluoric acid is highly dangerous to handle due to its corrosive and toxic properties. It can cause severe burns, tissue damage, and even be fatal if not handled properly.
?What precautions should be taken when working with hydrofluoric acid
When working with hydrofluoric acid, it is essential to wear appropriate personal protective equipment (PPE) such as gloves, goggles, and a lab coat. Adequate ventilation and engineering controls should be in place, and proper training on handling, storage, and emergency response procedures should be provided.
?What should I do in case of exposure to hydrofluoric acid
If exposed to HF acid, immediate action is crucial. Rinse the affected area with water for at least 15 minutes and seek medical attention immediately. It is important to follow specific first aid procedures for hydrofluoric acid exposure due to its unique toxicological effects.
? How should HF acid be stored and disposed of
Hydrofluoric acid should be stored in designated containers made of compatible materials, in well-ventilated areas, away from heat sources and incompatible substances. Disposal should be done according to local regulations, typically through specialized waste management facilities or services that can handle hazardous materials.