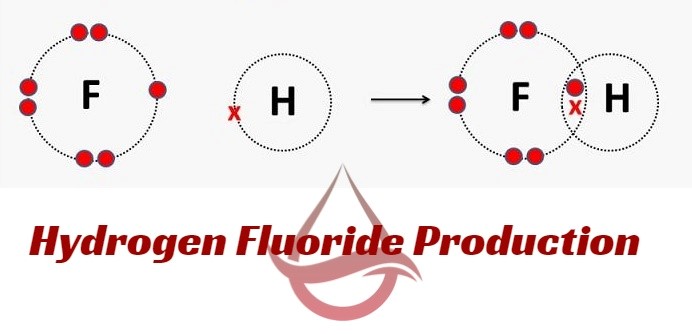
Definitions
Hydrogen fluoride (HF) is a highly useful chemical compound that is utilized in various industries, such as pharmaceuticals, petrochemicals, and electronics. The production process of hydrogen fluoride involves several steps that must be meticulously followed to ensure quality and safety. This essay will provide an overview of the hydrogen fluoride production process, highlighting the key steps involved.
1
The first step in the production of hydrogen fluoride is the extraction of naturally occurring minerals, such as fluorite or fluorspar. These minerals are typically found in countries like China, Mexico, and South Africa. Once extracted, the minerals undergo a purification process to remove impurities and yield a high-quality fluorite concentrate.
2
The next step involves the reaction of the purified fluorite concentrate with strong sulfuric acid to produce calcium sulfate and hydrogen fluoride. This reaction takes place in a reactor vessel equipped with heating and agitation mechanisms to ensure efficient mixing and heat transfer. The reaction is exothermic, resulting in the release of heat, which must be carefully managed to prevent any safety issues.
3
After the reaction is complete, the resulting mixture of calcium sulfate and hydrogen fluoride is cooled and subjected to a separation process. This process involves filtering the mixture to separate the solid calcium sulfate and collecting the liquid hydrogen fluoride. The collected hydrogen fluoride is then purified further by distillation to remove any residual impurities and obtain a highly pure product.
4
Once purified, the hydrogen fluoride can be stored in specialized containers or utilized in various industrial applications. It is essential to ensure proper handling, storage, and transportation of hydrogen fluoride, as it is a highly corrosive and dangerous substance. Adequate safety precautions must be in place to prevent accidental leaks or exposure to workers or the environment.
Byproducts
The production process of hydrogen fluoride also generates byproducts, such as calcium sulfate. These byproducts can be further processed or sold for other industrial applications, minimizing waste and maximizing resource utilization.
Moreover, it is crucial to note that the production of hydrogen fluoride requires significant energy consumption. Industries are continually striving to minimize energy usage and environmental impacts by implementing various energy-efficient technologies and adopting sustainable practices.
Furthermore, the production of hydrogen fluoride should also adhere to strict regulations and standards set by governing bodies to ensure environmental and worker safety. Proper waste management practices should be in place to dispose of any hazardous waste generated during the production process.
Final word
In conclusion, the production process of hydrogen fluoride involves the extraction of fluorite minerals, reaction with sulfuric acid, separation, purification, and storage of the resulting hydrogen fluoride. It is a complex process that requires careful handling, adherence to safety protocols, and consideration of environmental impacts. Continuous efforts are being made by industries to improve efficiency, minimize waste, and reduce energy consumption in the production of hydrogen fluoride.